Production
The technological asset of E-Pharma’s production plant is UNIQUE, DYNAMIC and INTEGRATED
DIMENSION
- 2 PLANTS 14340 m2
- PRODUCTION AREA 4690 m2
- WAREHOUSES 5740 m2
- LABS 870 m2
- OFFICES 1130 m2
FACILITIES
- 8 fluid bed granulators
- 9 tablets presses
- 6 bagging lines
- 2 stick pack lines
- 2 blister pack lines
- 1 strip pack line
- 5 tubes pack line
Each production line is automatic and equipped with:
- Dinamic scales (Bilance dinamiche)
- Metal detectors
- Packing machines (astucciatrice)
- BL serialization and aggregation

- Cartoning machines
FROM BULK TO READY FOR SALE PRODUCT in short-time and IN-LINE controlled process
With the collaboration of selected suppliers, we have created our own installation solutions to facilitate and better controll our production processes.
Our Technological and Analitical Lab is equipped with the same IN SCALE installation solution where the SCALING UP of the new formulations is granted.
We grant a TOP QUALITY level across the entire production life cycle
UTILITIES
HVAC SYSTEM and COMPRESSED AIR PRODUCTION AND DISTRIBUTION SYSTEM
All the production areas have ENVIRONMENTAL CONTROLLED conditions: temperature and humidity with average range of 10%RH-20C°
Warehouses temperature is set up below 25°C.
Osmosi purified water production and distribution.
Osmosi system - below 7 mSiemens - for te production and distribution of purified water to granulation and cleaning processes
The use of WATER as granulating agent is a key aspect of the technologies of E-Pharma which are solvent and alcohol free.
- HVAC SYSTEM and COMPRESSED AIR PRODUCTION AND DISTRIBUTION SYSTEM
- Osmosi purified water production and distribution
PERSONNEL
- Production -> 130
- QC -> 37
- QA -> 9
- Logistics -> 24
- Engineering -> 2
- Mainteinance -> 11
- Offices -> 65
TRAINING
Staff training program on GMP, PROCEDURES, SAFETY, PHARMACOVIGILANCE, NEW ROLES AND COACHING.
During 2022 we delivered 1006 hours on training courses, of which
- 85 on GMP
- 278 on regulatory updates
- 175 on safety procedures
- 309,5 on personell management (onboarding and coaching)
- 98,5 on company procedure and processes
- 63 on IT
PROCEDURE/DOCUMENTS
- Procedures are being managed by Adiuto validated software, which enable us to approve GMP functions-related documents as well as to monitor their expiry dates
- Non-conformity, deviation and customer/supplier complaint reports are also being managed by Adiuto with same approval flow
- Batch records are approved in compliance with our specific Quality System procedure
- Printed form documents are managed following criticality categories which imply different protection levels, such as the validation of the structure of the form or the protection of restricted cells where justified.
RAW AND PACKAGING MATERIALS
We grant the BUSINESS CONTINUITY per priority index and qualitative levels by a continuous materials scouting process.
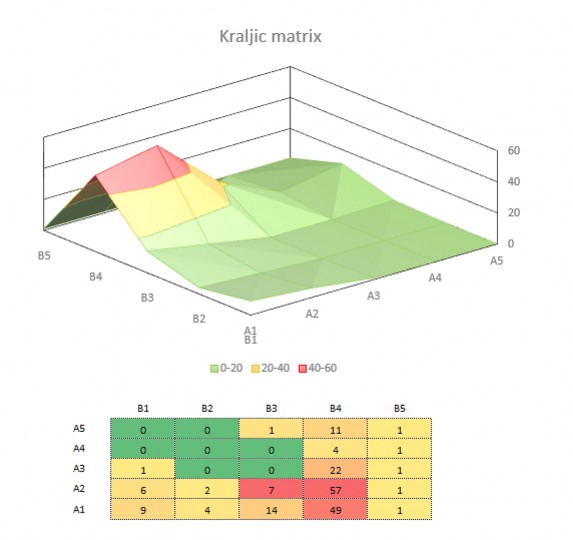
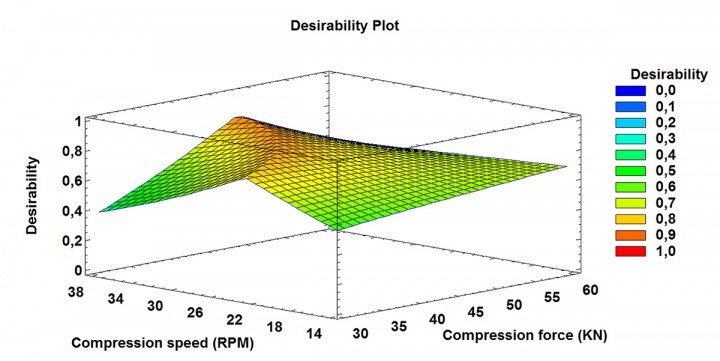
PARAMETERS
- We integrate Quality by Design principles to our manufacturing processes
WORK FLOWS
- We check, track and select the best operational work flows using IDEF modeling to grant quality and efficiency to our business activities

